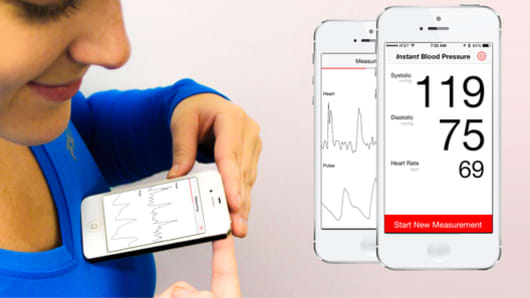
A blood pressure app downloaded by tens of thousands of people before sales halted last year gave many patients with hypertension the false impression that their vital signs were normal when in reality they were dangerously high, a U.S. study suggests.
The app in question, Instant Blood Pressure from AuraLife, was designed to estimate blood pressure by placing the top edge of a smartphone on the left side of the chest and holding the right index finger over the camera on the device.
But approximately four out of every five people with hypertension got inaccurate readings from the app that suggested their blood pressure was in a normal range when it was actually high, the study found.
“If Instant Blood Pressure worked, it would be a revolutionary new technology that would allow for low-cost screening and management of hypertension among smartphone users,” said lead study author Dr. Timothy Plante of Johns Hopkins University in Baltimore.
“That it doesn’t use a cuff is neat as folks don’t generally like carrying around a bulky blood pressure monitor,” Plante added by email. “Inaccurate measurements for high or low readings are concerning, however. Hypertension is known as the silent killer as it has an asymptomatic course that leads to serious conditions like heart disease, kidney disease, and stroke.”
At least 148,000 copies of the app were sold for $4.99 from June 2014 to July 2015, Plante and colleagues estimate in JAMA Cardiology.To test how well the app worked, the research team used the same standards new blood pressure cuffs must meet to win U.S. regulatory approval for use in doctors’ offices.
They enlisted 85 volunteers, more than half with a diagnosis of hypertension and nearly all on medications for the condition.Researchers checked blood pressure twice with the app, following the instructions provided by the developer, and then compared the average result from those two readings to a separate measurement from a traditional blood pressure cuff.
Healthy people have a systolic blood pressure, the measurement when the heart beats, of less than 120 mmHg, and diastolic pressure, when the heart rests, of less than 80 mmHg. Blood pressure above 140 mmHg systolic or 90 mmHg diastolic are considered hypertension.
The average differences between the app results and the traditional cuff were 12.4 mmHg for systolic blood pressure and 10.1 mmHg for diastolic blood pressure.Generally, the app overestimated low blood pressure and underestimated hypertension.
It’s hard to imagine any mobile app to measure blood pressure without a hardware component that does the job of a cuff, said Dr. Beverly Green, a researcher at Group Health Research Institute in Seattle who wasn’t involved in the study.
“I am not an engineer, but from what I understand, you need a cuff (the thing that wraps around your arm and is pumped up) preferably on the upper arm, to get an accurate BP,” Green said by email.
“The cuff compresses the arteries and measures the amount of pressure the heart needs to get blood out to the arteries and the pressure of the arteries when the heart is relaxed,” Green added.
The app was available in both Apple’s App Store and Google Play. Even though it’s no longer sold, it’s possible some users still have it on their phones. Apple confirmed the app was rejected, while Google declined to comment.
Ryan Archdeacon, CEO and co-founder of AuraLife, told Reuters Health by email that several issues with the study “render it invalid.” Among these, multiple updates to the app during the study period and afterward improved the accuracy of readings by 30 percent, according to Archdeacon.
Moreover, the app was not designed to measure blood pressure above 158 mmHg systolic or 99 mmHg diastolic, he said. “Instant Blood Pressure is not a medical device and is not intended to diagnose disease, including hypertension,” Archdeacon said.
Apps have potential to help more than 1 billion patients with hypertension worldwide manage their condition, though the findings highlight the pitfalls of consumers using a nascent technology, said Nilay Kumar, a researcher at Harvard University who wasn’t involved in the study.